

|
- | Einsteinium [1] is a synthetic radioactive isotope, first discovered by Albert Ghiorso and co-workers in debris from a 1952 thermonuclear explosion in the South Pacific. It is produced by intense neutron irradiation of Uranium-238 (U-238). There are 11 known isotopes. |
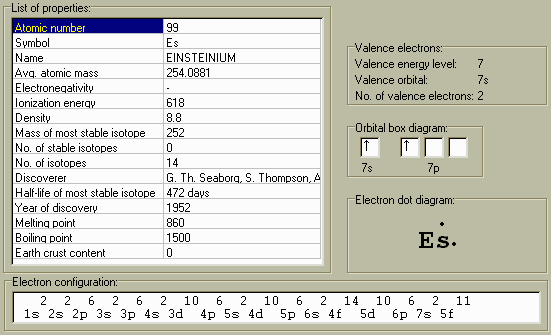
Einsteinium
[4]
Pronounced As: instinm, insti-
[named for Albert Einstein
[5]],
artificially produced
radioactive
chemical element;
symbol: Es;
Atomic#: 99;
mass of most stable
Atomic#
isotope: 252;
The Melting Point is about 860?C;
The Boiling Point is unknown;
The Specific Gravity (density) is unknown;
valence
+2, +3.
Einsteinium
[6],
is a member of group IIIb of the
Periodic Table;
its chemical properties are believed to be similar to those of the other members of the
actinide series.
The seventh
transuranium element
to be discovered,
Einsteinium
was isolated in Dec., 1952, by Albert Ghiorso
and his co-workers at the Univ. of California at Berkeley in residue from
the first thermonuclear test explosion in the South Pacific.
They identified Einsteinium-253, which has a
half-life
of 20.5 days.
It was not until 1961 that a weighable quantity (about 0.01 microgram)
of the element was separated;
it was used to prepare the element
mendelevium.
Weighable quantities of
einsteinium
have since been prepared by neutron bombardment of
plutonium.
Seventeen
isotopes,
all of which are
radioactive,
are known.
Einsteinium-252, the most stable
isotope,
has a
half-life
of 472 days.
